A new generation of “smart” implantable devices could replace traditional medication to treat a range of chronic conditions, including cardiac disease.
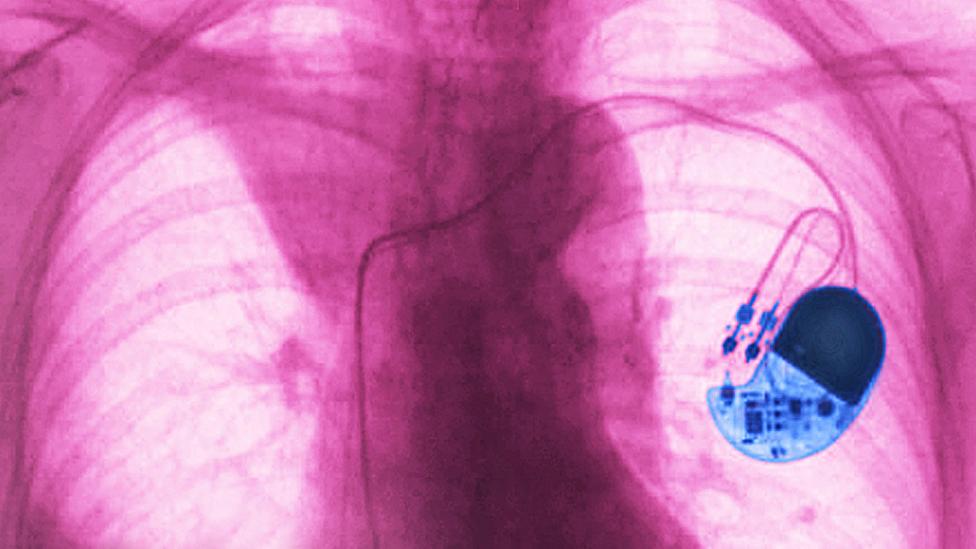
The modern pacemaker is a medical marvel. No bigger than a matchbox, this implanted device sends electrical pulses to the human heart to keep it beating regularly. Globally, 1.25 million pacemakers are fitted each year, vastly improving the quality of life for patients whose hearts beat abnormally and restoring life expectancies to normal levels for some individuals.
While the physical device has steadily improved over the last few decades since the first totally implantable pacemaker was fitted in 1958, the basic idea behind a pacemaker has not changed. Implanted electrodes monitor your heartbeat and if it becomes abnormal, the device can deliver electrical pulses to trigger your heart muscles to contract so they continue to pump blood around your body. Whether you’re asleep or running a marathon, the pacemaker should keep your heart reliably beating with the same rhythm.
But there are some who think the pacemaker could do so much more.
Rather than simply jolting our heart tissue into action when it fails to beat by itself, implantable devices could monitor and diagnose the signs of disease, help to manage chronic conditions and even provide new types of treatment that we could simply download like an app on our phone.
By tapping directly into the network of nerves that run around our bodies, a new generation of pacemaker-like devices could be used to tackle diabetes, arthritis, and Parkinson’s disease, as well as improve bladder control and offer better pain management.
So, how can we make pacemakers better?
The human body houses its own natural pacemaker – a network of biological wires we call nerves that send electrical signals, along with chemical messages, to all of the major organs and tissues in your body. In the past 20 years, a field known as bioelectronics has been attempting to directly tap into these signals.
Bioelectronic devices are an evolution from the pacemaker industry. They attempt to replicate neural signals, interfacing with the body to control activity in dysfunctional neural circuits that give rise to disease. Deep brain stimulators are a well-known example of such devices and have been used to help control the tremors, rigidity and movement problems associated with Parkinson’s disease by sending an electrical signal into the brain. Neurostimulation devices have also been used to treat conditions such as epilepsy in situations where drug treatment has failed.
But, today’s bioelectronic implants are blunt instruments, which do not take into account some important changes in our bodies. This is because scientists have struggled to understand the exact neural signal patterns – known as neural biomarkers – that affect our health.
Once we can understand neural signals, we can effectively “talk back” to our body using implantable devices to autonomously deliver treatments
If you think of this information as a language, then biomarkers are the individual words. Neural biomarkers are one type of biomarker. As we understand more of them, we can start to understand the language of the human nervous system and what it’s telling us about our body.
That’s not all. Once we can understand neural signals, we can effectively “talk back” to our body using implantable devices to autonomously deliver treatments to the patient through the stimulation of specific nerves.
The attraction of an implantable device is easy to understand. When working correctly it should automatically monitor and deliver treatment without the patient even being aware.

This can help to avoid problems such as non-adherence to prescribed medications. Research reveals that, in the US alone, 125,000 deaths and at least 10% of hospitalisations are caused by patients not taking the recommended dose of their medication.
By decoding the messages passing through your nerves and reacting to that information, next-generation implantable devices should be able to monitor conditions and provide treatment as it is needed.
For example, if you do some strenuous exercise, then the device picks up this change from your nerve signals and increases your heartbeat to match the level of your activity.
Or, if your heartbeat needs to gradually slow as you age, the implantable will match this physiological change. Furthermore, it could alert a clinician if a cardiac disease is progressing or emerges in your body, allowing them to make a proactive medical prognosis.
We need more neural data and better interpretation techniques so we can listen to the right words needed to understand the language of the human body
This is no mean feat, requiring the implantable device to decode your neural messages and react in real-time by passing another message to your brain, causing the target organ to react.
But neural data is incredibly noisy and complex. The human heart does not exist alone, its function is affected by other factors and messages coming from other organs, such as how fast you’re breathing, what you’ve just eaten and so on.
To accurately decode all this information, we need more neural data and better interpretation techniques so we can listen to the right words needed to understand the language of the human body. Bios have turned to a form of artificial intelligence known as machine learning to help them do this.
The team has been recording raw neural data through neural interfaces, which Bios has developed, placing them alongside recordings of physiological signals such as heart rate, blood pressure, glucose levels, body temperature and levels of physical activity. They have been able to synchronise months of continuous neural and physiological data on timescales long enough for their AI algorithms to identify patterns that point towards persistent neural biomarkers, and how they relate to changes in organ function.

As the algorithm learns more from an individual, it can personalise its automated responses to each patient’s needs. These “bidirectional neural interfaces” can also build up a clinical picture of the patient over time and map how a patient’s disease is progressing, allowing clinicians to create personalised care plans for each individual.
“We are essentially looking at how you can deliver responsive treatments for chronic conditions through algorithms via the nervous system instead of relying solely on pharmaceuticals,” Armitage adds.
Bios’ implantables will be used in human clinical trials over the next few years, initially in upper-body amputees. This is because the neural signals required to move an arm are easier to assess and less complex, compared to those required to maintain a healthy heart – or lung, or pancreas.
Armitage predicts, however, that we will see “personalised responsive devices in the next three to five years” that could treat a range of conditions, including hypertension, diabetes, bladder control and chronic pain.
Ultimately, Bios is hoping its technology will provide a platform that clinicians will be able to build upon to develop new treatments. For example, a clinician could build a neural treatment for a specific condition much as they would a mobile phone app that could run on the Bios platform, breaking down a chronic condition into nothing more than an algorithm.
“The technology we are developing is really a platform for reading and writing to the nervous system so the condition we treat is then an algorithm,” says Armitage.

Decoding the heart
But key to this approach is the ability to break diseases down into algorithms in the first place. Scientists are harnessing machine learning to understand how a wide range of biomarkers signals – such as hormones or specific biochemicals in the blood – could indicate specific conditions for the heart and other organs.
For example, diabetic patients are at least twice as likely as non-diabetics to die from heart disease. But, while we know diabetes harms the heart and sabotages its ability to make energy at the cellular level, we still do not know exactly why this is the case.
A team at West Virginia University recently investigated the biomarkers associated with diabetes using machine learning algorithms to search for proteins and metabolic signals in tissue samples taken from diabetic and non-diabetic patients.
“Initially, we found that there were no clear distinctions between the diabetic and the control cohorts,” says Quincy Hathaway, a medical student at the university who conducted the research as part of his doctoral thesis. “But using machine learning algorithms provided the sensitivity to tease out more details and identify a unique signature.”
His work could lead to better diagnostics and treatments for diabetic patients with cardiovascular complications. A clinician could examine a tissue sample and identify particular cardiac diseases from these distinctive biomarkers. If we could pinpoint the biomarkers with enough accuracy in the future, a simple blood test could work out whether a patient has diabetes and is at risk of developing a cardiac condition.
The next step is to continue to train the machine learning algorithms with more data. But this is not without its challenges, as Hathaway adds: “It would take thousands of patients to find a distinctive marker using deep sequencing on every genomic level.”

“It’s not actually the acquiring of the data that’s the hard part, it’s understanding what the data means in a larger context,” he says.
But there are many who see the potential of this approach. In the US, for example, the National Institutes of Health’s Stimulating Peripheral Activity to Relieve Conditions (SPARC) program is investigating the development of therapeutic devices that modulate electrical activity in nerves to improve function across a wide range of organs.
Researchers predict some next-generation adaptive deep brain stimulation devices that target specific conditions could be available in two or three years. Among the most promising of these are being developed to treat neurological conditions, such as Parkinson’s Disease.
Helen Bronte-Stewart, director of the Stanford Movement Disorders Center, and her team are working on an adaptive deep brain stimulation system for the condition that uses a sensor worn on the wrist to detect symptoms including a freezing gait and tremor. This wristband is linked to an implant in the brain through Bluetooth technology, which produces tiny electrical signals in the patient’s brain according to their symptoms in near real-time.
To accurately monitor and treat diseases in this way, individual neurons need to be tapped with incredible precision
“We now know a lot more about the relevant neural signals than we did a few years’ ago,” says Bronte-Stewart.
But to accurately monitor and treat diseases in this way, individual neurons need to be tapped with incredible precision. Current electrode technology makes this difficult as different electrodes are used to stimulate and record signals.
One team of researchers at the University of Melbourne, however, are using diamond-coated carbon fibres in the hope of creating arrays of electrodes that can do both jobs for individual neurons.
“With our electrodes, I see an opportunity to address debilitating diseases such as Parkinson’s, dementia, chronic pain and, maybe even, depression,” says Melanie Stamp, who is part of the research team at the University of Melbourne. While the carbon fibres should also be biocompatible, meaning they reduce the risk of the patient’s body rejecting the implant, Stamp cautions they are still to perform any clinical trials, which will be an important test of the new electrodes performance and safety.
And this highlights an important point for all implantable devices. The invasive nature of surgically attaching electrical devices to nerves means they need to be rigorous assessed to ensure they will not trigger any harmful, unintended reactions from patient’s bodies.

“For instance, brain stimulation techniques with focused ultrasound are being developed, that do not need electrodes to be implanted into the brain,” explains Fleur Zeldenrust, a neurophysiologist at Radboud University in the Netherlands who studies how the structure of the brain links to its function. “This can, of course, be a great help to patients, as implantation of electrodes always includes some risk. But this also means it becomes easier to manipulate brain activity, with all the ethical implications that come with that.”
There may, however, be limitations to how many conditions can be treated. The brain does not exist in silos, but is a highly distributed and interlinked system, adds Zeldenrust.
“The processing that’s being done in our brain is very distributed,” she says “But a deep brain stimulation electrode influences all cells surrounding one location simultaneously and that will only activate the neurons around it.”
This means treating a broader range of symptoms caused by complex disorders will be difficult as some neurons would need to be stimulated but not others at multiple locations at different times. This is not possible at the moment.
But despite the many technical and regulatory hurdles that still exist, a recent report from The Royal Society in the UK concluded that the next generation of neural interfaces implants could bring wide-ranging benefits.
“In medicine, neural technologies are set to mature and expand significantly in the coming decades, potentially overtaking pharmaceuticals in efficacy in some areas,” it says.
It raises the possibility that a visit in the doctor could soon end with us downloading an algorithm rather than popping pills to make ourselves feel better.
Credit: Source Link